Gut Microbiota Dysbiosis: A growing body of scientific research is shedding new light on the connection between gut health and eye diseases, particularly diabetic retinopathy (DR). According to a 2026 systematic review and meta-analysis, gut microbiota dysbiosis — an imbalance in the community of microorganisms living in the digestive tract — may play a significant role in the development and progression of diabetic retinopathy. The findings strengthen the emerging concept of the “gut-eye axis,” a biological pathway linking intestinal health to ocular function.
As diabetes rates continue to rise worldwide, understanding the broader systemic effects of the disease has become increasingly important. Diabetic retinopathy remains one of the most serious complications of diabetes and a leading cause of preventable blindness among working-age adults. Researchers now believe that disturbances in gut microbiota may contribute to the inflammatory and vascular changes that damage the retina in diabetic individuals.
Diabetes and the Growing Burden of Diabetic Retinopathy
The World Health Organization (WHO) projects that by 2045, one in 10 people in the WHO European Region will be living with diabetes. Globally, the condition is increasing at an alarming rate due to lifestyle changes, aging populations, and rising obesity levels.
Diabetic retinopathy is a microvascular complication of diabetes that affects the retina — the light-sensitive tissue at the back of the eye. It typically develops after prolonged periods of uncontrolled blood sugar levels. Over time, high glucose levels damage the tiny blood vessels in the retina, leading to leakage, swelling, abnormal vessel growth, and potentially permanent vision loss.
DR is considered one of the leading causes of preventable vision impairment in adults aged 20 to 74 years. Despite improvements in screening and treatment, many patients are diagnosed at advanced stages, when visual damage may already be irreversible.
The Gut-Eye Axis: A New Frontier in Research
Traditionally, diabetic retinopathy has been understood primarily as a result of hyperglycemia-induced vascular damage. However, researchers are now exploring how systemic inflammation and metabolic changes — influenced by gut microbiota — may also play a role.
The human gut hosts trillions of bacteria, viruses, and fungi collectively known as the gut microbiota. These microorganisms help regulate digestion, immune responses, metabolism, and even neurological function. When the balance of beneficial and harmful microbes is disrupted — a condition known as dysbiosis — it can trigger chronic inflammation and metabolic disturbances.
The concept of the “gut-eye axis” describes a bidirectional communication pathway between the intestinal microbiome and ocular tissues. Through this axis, microbial metabolites, inflammatory molecules, and immune signals can influence retinal health. Conversely, systemic diseases like diabetes can alter gut microbial composition, further complicating the disease process.
Emerging evidence suggests that gut dysbiosis may contribute to retinal damage by:
- Increasing systemic inflammation
- Promoting vascular dysfunction
- Allowing translocation of pro-inflammatory metabolites into circulation
- Disrupting immune regulation
Findings from the 2026 Systematic Review and Meta-Analysis
The 2026 systematic review and meta-analysis evaluated 18 studies, including observational research, cohort studies, and Mendelian randomisation analyses. These studies compared gut microbiota profiles among patients with diabetic retinopathy, patients with diabetes without retinopathy, and healthy individuals.
Also read: Eating Healthy Doesnt Have to Cost a Fortune
Alpha Diversity Results
Alpha diversity refers to the diversity of microbial species within a single sample. Interestingly, the meta-analysis found no statistically significant differences in alpha diversity between:
- Patients with diabetic retinopathy
- Patients with diabetes
- Healthy controls
This suggests that the overall number and richness of microbial species may not drastically change across groups.
Beta Diversity Results
However, beta diversity — which measures differences in microbial composition between groups — revealed more striking findings. Researchers observed consistent “distinct clustering of microbial communities” among the three populations.
In simpler terms, while the number of microbial species may not differ dramatically, the specific types and proportions of bacteria present vary significantly between those with DR, those with diabetes alone, and healthy individuals.
This distinct microbial pattern supports the theory that gut dysbiosis may contribute to the progression of diabetic retinopathy.
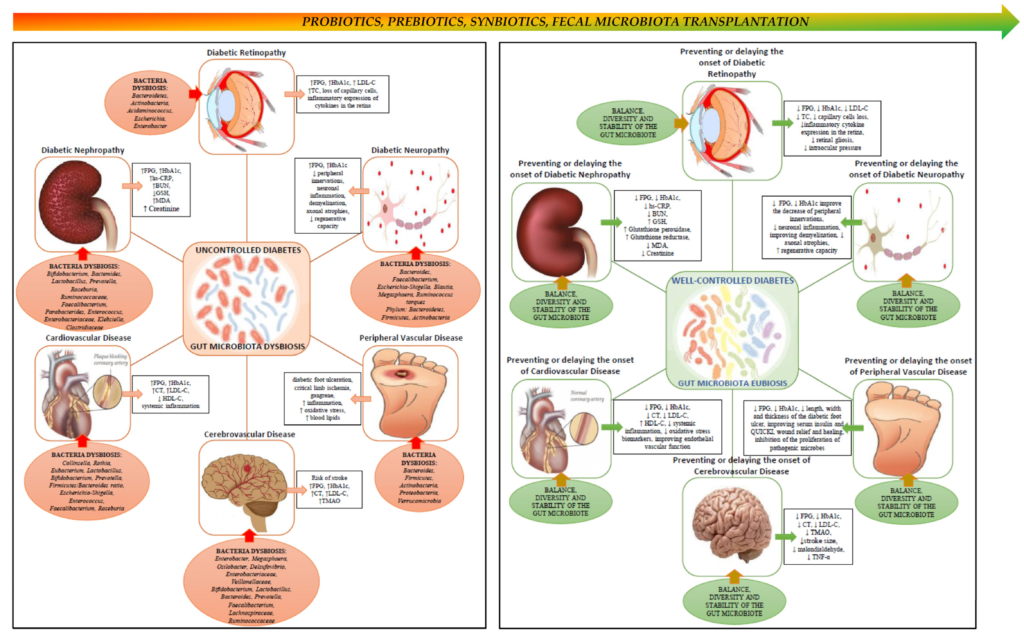
How Gut Dysbiosis May Worsen Diabetic Retinopathy
The review suggests that gut dysbiosis may exacerbate diabetic retinopathy through several mechanisms:
1. Translocation of Pro-Inflammatory Metabolites
When gut barrier integrity is compromised, harmful bacterial byproducts such as lipopolysaccharides (LPS) can enter the bloodstream. These molecules trigger systemic inflammation, which may damage retinal blood vessels.
2. Chronic Low-Grade Inflammation
Persistent inflammation is a known driver of diabetic complications. Dysbiosis may amplify inflammatory pathways that accelerate retinal nerve cell damage.
3. Vascular Integrity Disruption
Microbial metabolites can influence endothelial function — the health of blood vessel linings — potentially worsening the microvascular damage characteristic of DR.
Together, these mechanisms support the gut-eye axis hypothesis and suggest that gut microbial imbalance is not merely a consequence of diabetes but may actively contribute to retinal disease progression.
Limitations of the Research
Despite promising findings, the researchers acknowledged several limitations:
- Inconsistency in alpha diversity measures across studies
- Predominantly Asian cohorts, which may limit global generalisability
- Methodological variations in microbiome analysis techniques
- Differences in diet, lifestyle, and genetic backgrounds among study populations
These factors highlight the need for larger, more diverse, and standardised studies to confirm the association between gut dysbiosis and diabetic retinopathy.
Clinical Implications and Future Directions
The identification of gut dysbiosis as a contributing factor in diabetic retinopathy opens new avenues for prevention and treatment.
Researchers suggest several potential interventions aimed at restoring microbial balance:
Dietary Modifications
High-fiber, plant-based diets support beneficial bacteria and reduce systemic inflammation. Reducing ultra-processed foods may also help maintain microbial diversity.
Probiotics and Prebiotics
Targeted probiotic supplementation may help restore beneficial bacterial strains. Prebiotics — non-digestible fibers that feed good bacteria — may further enhance gut health.
Faecal Microbiota Transplantation (FMT)
Although still experimental in the context of diabetic retinopathy, FMT involves transferring healthy gut bacteria from a donor to restore microbial balance.
While these interventions show promise, clinical trials are necessary to determine their effectiveness specifically for preventing or slowing diabetic retinopathy progression.
Read about: Fitness Trainer Reveals How He Modified His Tamil Diet for Weight Loss
Conclusion
The 2026 systematic review and meta-analysis strengthens the growing evidence linking gut microbiota dysbiosis to diabetic retinopathy. While overall microbial diversity may not differ significantly, distinct shifts in microbial composition appear to be associated with the disease.
The concept of the gut-eye axis offers a compelling explanation for how intestinal health influences retinal damage in diabetes. By addressing gut dysbiosis through dietary, probiotic, or emerging therapeutic strategies, clinicians may one day complement traditional glucose control approaches with microbiome-focused interventions.
As diabetes prevalence continues to rise globally, understanding these interconnected systems will be crucial in developing more holistic and effective prevention strategies for vision-threatening complications like diabetic retinopathy.